Exploring The Potential of You: Understanding pH Levels - Potential Hydrogen.
ROAR 🌟 Today, we’re embarking on an exciting adventure into the vibrant world of potential hydrogen, or pH! Grab your explorer hats because we’re about to uncover how this often-overlooked element plays a pivotal role in our lives and our beautiful planet.
Imagine sipping on a refreshing drink that revitalizes you, or enjoying the crispness of freshly washed fruits and veggies—all thanks to the wonders of pH balance! Our hydration and health are not just routine concerns; they’re essential elements of our daily joy and well-being. By understanding pH, we can make informed choices that enhance our lives, nurture our bodies, and promote a healthier environment.
Let's journey through the science with a sprinkle of fun! What exactly is pH? It’s a scale that measures how acidic or alkaline a substance is, and trust me, its impacts are far-reaching. From the water we drink to the soil nurturing our crops, pH levels influence everything, including our precious ecosystems. 🌍
As we dive deeper into this vibrant topic, we’ll discover tips and tricks for maintaining the perfect pH balance in our lives. Whether that means adjusting the pH of our drinking water for optimum hydration, learning about the best pH levels for our garden, or even exploring how our diets can help us achieve that balance—there's so much joy to be found in this delightful exploration!
So, let's celebrate the beauty of pH together and understand its importance in creating a healthier, happier world. Ready to blaze the trail into this captivating journey? Let’s soar through the science and sprinkle in the joy! 🎉✨
What is pH?
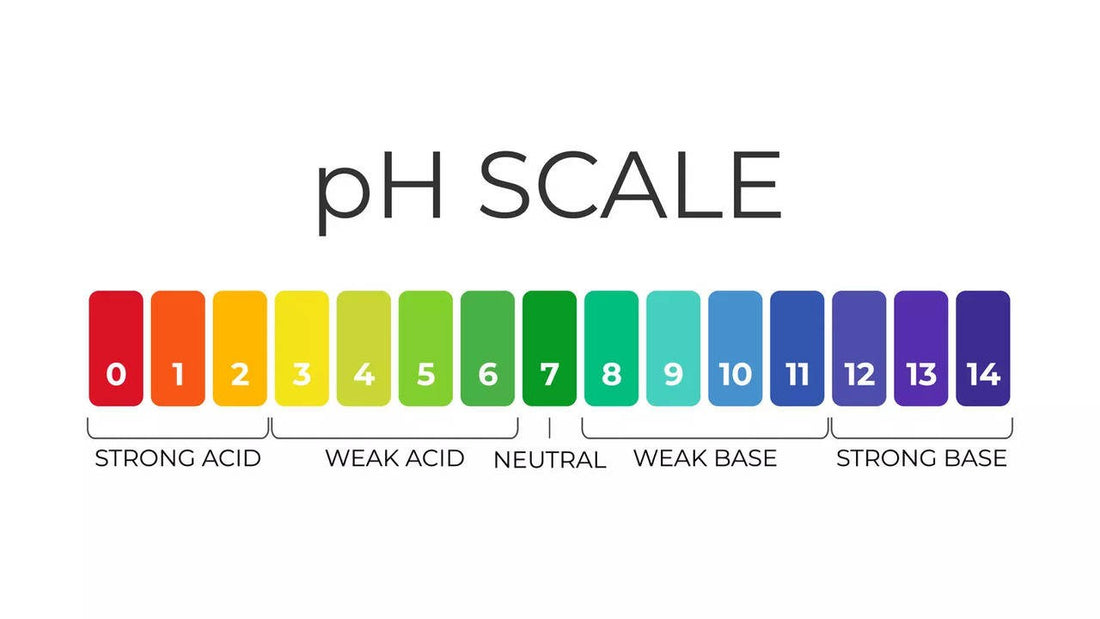
At its essence, pH is a quantitative measure of the acidity or alkalinity of a solution, expressed on a logarithmic scale ranging from 0 to 14. This scale is pivotal in chemistry, environmental science, agriculture, and health sectors. A pH of 7 indicates a neutral solution—like our beloved pure water—neither acidic nor alkaline. When the pH drops below 7, we enter the realm of acidity, while values above 7 signify increasing alkalinity.
The formula for pH is given by the negative logarithm of the hydrogen ion concentration \([H^+]\) in moles per litre:
\[
\text{pH} = -\log[H^+]
\]
As we sip on refreshing beverages like lemonade, let’s appreciate the role of hydrogen ions in both flavor and nutrient uptake. Acids, characterized by their release of hydrogen ions in solution, contribute to the tartness we taste. This acidity can also play a crucial role in nutrient availability for plants. In soil, hydrogen ions help to dissolve nutrients, making them more accessible to plant roots.
On the flip side, bases or alkaline substances can enhance sweetness and smoothness, balancing flavors. In the context of nutrient uptake, alkaline conditions can influence the solubility of certain nutrients, promoting or hindering their absorption by plants. For instance, some nutrients are more readily available in slightly acidic conditions, while others thrive in more alkaline environments.
Ultimately, maintaining the right pH balance—affected by the concentration of hydrogen ions—ensures that plants can effectively absorb the nutrients they need for optimal growth and health.

4. Crop Examples and pH Sensitivity
Understanding the pH of soil is essential not only for general agricultural practices but also for optimizing the growth of specific crops. When it comes to farming, it's truly like a dance with nature, where every step counts! Different crops have varying pH preferences, each requiring unique care and attention to flourish and yield bountiful harvests.
In Australia, the diverse climate and soil types make it even more crucial to tailor our techniques to suit the specific needs of each crop. Let's take hemp, for instance, which is gaining popularity not just for its versatile uses but also for its impressive adaptability. Industrial Hemp thrives in a pH range of about 6 to 7.5, and growers must carefully manage soil pH to create the perfect environment for this incredible plant to flourish.
Other crops, like citrus trees, prefer a slightly more acidic soil, with a pH range of 5.5 to 6.5. On the other hand, legumes, which are fantastic for enriching the soil with nitrogen, are happiest in a pH range of 6 to 7. This variation highlights the importance of understanding local agricultural standards— after all, it's not just about planting seeds; it's about nurturing the right conditions to see them thrive!
Moreover, incorporating pH management techniques can open up a treasure trove of possibilities in agricultural practices across different cultures. For instance, some traditional methods from Indigenous Australian cultures emphasize the connection between soil health and crop yield, and this knowledge can beautifully complement modern scientific approaches.
By adjusting our techniques—whether it’s through soil amendments, crop rotation, or careful monitoring—we can honor the wisdom of these practices while optimizing our harvests. In a land as rich and diverse as Australia, embracing these variations can lead to sustainable and abundant farming practices that benefit both our ecosystems and our communities.
Hemp (Cannabis sativa)
Hemp is gaining popularity in Australia due to its versatility and numerous uses, including textiles, food, and bioplastics. Hemp prefers a slightly acidic to neutral soil pH, ideally between 6.0 and 7.0. This pH range enhances nutrient uptake, which is critical for the optimal growth of hemp plants. Farmers often conduct soil tests to adjust pH through amendments, ensuring healthy crops and maximising yield.
Wheat (Triticum spp.)
Wheat is one of Australia’s major crops, and its optimal growth occurs in soils with a pH range of 6.0 to 7.5. Wheat plants can tolerate a slightly wider pH range, but soil that is too acidic can lead to nutrient deficiencies, particularly in phosphorus. Australian farmers often utilize practices such as liming to maintain pH levels within the desired range, promoting strong, healthy wheat crops.
Potatoes (Solanum tuberosum)
Potatoes prefer a soil pH between 5.5 and 6.5. Acidic soils can hinder tuber development and increase susceptibility to diseases such as scab. To achieve the best results, potato growers often implement soil amendments based on pH tests and may choose to lighten overly acidic soils to promote optimal growth conditions.
Canola (Brassica napus)
Canola thrives in soils with a pH ranging from 6.0 to 8.0. Its resilience to a slightly wider pH range makes it a favorable option for many Australian farmers. However, ensuring that the pH remains within this range is still crucial for nutrient availability, particularly for essential micronutrients like boron and manganese.
Citrus (Citrus spp.)
Citrus trees, including oranges and lemons, prefer a slightly acidic to neutral pH, ideally between 6.0 and 7.0. Maintaining appropriate pH levels helps in preventing nutrient deficiencies and diseases. Australian citrus growers often adjust their soil's pH to this optimal range to support healthy fruit development and enhance production quality.
Why is pH Important?
Understanding pH is essential not just for our beverages but also for various aspects of our lives, including gardening, cooking, and even cleaning! Let’s explore some key areas where pH levels play a crucial role:
1. Gardening and Soil Health:
Plants thrive in specific pH conditions that significantly affect their nutrient availability and overall health, which we've given some examples of above! Supporting optimal nutrient uptake. For example, at a pH of 6.5, essential nutrients like nitrogen, phosphorus, and potassium are readily available to plants. Conversely, extreme pH values can lead to nutrient deficiencies or toxicities, inhibiting growth. Regular soil testing can help gardeners make informed adjustments using amendments like lime to raise pH or sulphur to lower it, ensuring that plants receive the nutrient balance they need for vibrant growth.
2. Water Quality:
Water quality is significantly influenced by pH, with Australian standards stipulating that drinking water should generally have a pH between 6.5 and 8.5. This range is critical for several reasons: it ensures the solubility and availability of essential minerals, reduces the risk of harmful contaminants, and preserves the sensory qualities of water, such as taste and clarity. Monitoring natural water sources' pH levels is also vital for ecosystem health, as aquatic organisms exhibit specific pH tolerances, influencing biodiversity and overall water quality.
3. Cooking and Baking:
The role of pH in cooking extends beyond flavour enhancement. The acidity of ingredients like lemon juice (with a pH of around 2) can facilitate Maillard reactions, contributing to browning and the development of complex flavours in dishes. In baking, understanding the pH of leavening agents is crucial; for instance, baking soda (sodium bicarbonate) functions optimally in a slightly acidic environment, while baking powder contains both an acid and a base, allowing for versatile recipes. Mastering pH can elevate culinary skills, transforming meals into harmonious experiences.
4. Health and Well-being:
Our bodies maintain a carefully regulated pH balance, with blood typically resting at a slightly alkaline level (around pH 7.4). This balance is vital for enzyme activity, metabolic processes, and overall physiological function. Diets rich in fruits, vegetables, and whole grains support this balance by providing alkalizing minerals, while excessive consumption of processed foods may lead to an acidic environment, potentially resulting in various health issues, including inflammation and reduced immunity. Prioritising a balanced diet encourages optimal well-being.
5. Plants, Fungi, and Symbiotic Relationships:
The interconnectedness of plants and fungi is vital for ecosystem health and nutrient cycling. Mycorrhizal fungi form symbiotic relationships with plant roots, enhancing nutrient uptake, especially phosphorus, in exchange for carbohydrates. The pH of the soil significantly influences this relationship. Most mycorrhizal fungi thrive in slightly acidic to neutral soil conditions (pH 6 to 7). When pH levels are imbalanced, it can adversely affect fungal viability and, consequently, the plant's access to essential nutrients. This delicate balance supports biodiverse ecosystems, improves soil structure, and promotes plant resilience against pathogens. By understanding and managing soil pH, gardeners can foster healthy mycorrhizal relationships, leading to flourishing plants and a vibrant garden ecosystem.
The Importance of pH
Understanding pH is fundamental not only in the realm of beverages but also across various facets of life, including gardening, cooking, and cleaning. Let’s delve deeper into the critical areas where pH levels significantly impact our daily activities:
1. Gardening and Soil Health:
The pH level of soil greatly influences the health of plants, nutrient availability, and overall growth. Most vegetables in home gardens thrive in slightly acidic to neutral pH levels, ideally within the range of 6 to 7. At a pH of around 6.5, essential nutrients—such as nitrogen, phosphorus, and potassium—are at their most bioavailable, enabling optimal nutrient uptake. Extreme pH conditions, either too acidic or too alkaline, can result in nutrient deficiencies or toxicities that inhibit plant growth. Regular soil testing is crucial for gardeners, allowing them to make informed adjustments with amendments like dolomitic lime to increase pH or sulfur to decrease it, thereby ensuring a favorable environment for robust plant development.
2. Water Quality:
Water quality is profoundly influenced by its pH, with Australian drinking water standards recommending a pH range of 6.5 to 8.5. This specified range is essential for several reasons: it promotes the solubility and availability of essential minerals, minimizes the presence of harmful contaminants, and maintains the sensory qualities of water, such as taste and clarity. Monitoring the pH levels of natural water sources is also vital for ecosystem health, as aquatic organisms exhibit specific pH tolerances that can influence biodiversity and the overall quality of aquatic habitats.
3. Cooking and Baking:
The significance of pH in culinary practices extends beyond mere flavor enhancement. The acidity of certain ingredients, such as lemon juice (approximately pH 2), impacts chemical reactions like the Maillard reaction, which contributes to browning and the development of complex flavors in various dishes. In baking, comprehending the pH of leavening agents becomes crucial; for instance, baking soda (sodium bicarbonate) is most effective in a slightly acidic environment, while baking powder contains both an acid and a base, enabling versatile recipes. Mastering the nuances of pH can elevate culinary finesse, transforming meals into intricate and enjoyable experiences.
4. Health and Well-being:
The human body maintains a delicate pH balance, typically resting at a slightly alkaline level around pH 7.4. This balance is essential for optimal enzyme functionality, metabolic processes, and various physiological functions. Diets rich in fruits, vegetables, and whole grains contribute to maintaining this pH balance by providing alkalising minerals. Conversely, excessive consumption of processed foods can create an acidic environment that may lead to health issues such as inflammation and compromised immunity. Prioritising a balanced diet rich in whole foods encourages overall well-being and health.
5. Plants, Fungi, and Symbiotic Relationships:
The symbiotic relationship between plants and mycorrhizal fungi is crucial for nutrient cycling and ecosystem health. Mycorrhizal fungi enhance nutrient uptake—particularly phosphorus—by forming beneficial associations with plant roots, while receiving carbohydrates in return. The soil pH plays a pivotal role in supporting this relationship, with most mycorrhizal fungi thriving in slightly acidic to neutral conditions (pH 6 to 7). An imbalance in pH levels can adversely affect fungal viability, subsequently limiting the plant's access to essential nutrients. Understanding and managing soil pH fosters healthy mycorrhizal relationships, leading to flourishing plants and vibrant garden ecosystems.
Measuring and Managing pH
For those eager to experiment, measuring pH has become increasingly accessible. Affordable pH test strips and digital meters provide quick and reliable readings for water, soil, and even culinary preparations. If your soil tests reveal an overly acidic condition, dolomitic lime can effectively raise pH levels, while elemental sulphur can be employed to lower alkaline soils. Embracing these practices encourages a hands-on approach to garden management and improving water quality, making every gardener a true steward of their environment!
Conclusion - The Final Paw
In conclusion, the exploration of pH, or potential hydrogen, transcends mere scientific inquiry; it enriches our lives in numerous ways. From maintaining our gardens and enhancing our cooking to ensuring clean water access and supporting our health, pH plays a vital role in sustaining balance and vitality.
So, whether you’re savoring a refreshing beverage, nurturing a flourishing garden, or creating culinary masterpieces, take a moment to appreciate the intricate science of pH. Here’s to a happier, healthier, and more balanced life!
And a heartfelt thank you for taking the time to read this article! Your interest in understanding and managing pH is a wonderful step toward cultivating not just your garden, but also a vibrant community focused on sustainability. If you're looking for more guidance on the best tools for various gardening tasks, don’t forget to check out our Soil Structure blog! It’s filled with insights on what tools to use for each job, ensuring you have everything you need to nurture your green space effectively.
Let’s keep the dialogue going! What’s your favourite pH-related tip or experience? Share your insights below! We can all learn from each other as we embark on this journey of growth and discovery!
Much Love, Ya Burr 🐻